Print Page | Close Page |
Home
Plant Science 135: 11 - 16.
Presence of proanthocyanidins in mutant green Sarracenia indicate blockage in late anthocyanin biosynthesis between leucocyanidin and pseudobase
Philip M. Sheridan a,b,*, Richard R. Mills a
a Department of Biology, Virginia Commonwealth University, Richmond, VA 23284-842012, USA
b Meadowview Biological Research Station, 8390 Fredericksburg Tnpk., Woodford, VA 22580, USA
Received 25 July 1997; received in revised form 20 March 1998; accepted 20 March 1998
Abstract
The insectivorous pitcher plant genus Sarracenia contains several species with mutants which lack red pigment in leaves, flowers and growth point. Mutants and wild-type contain various glycosides of the flavonols quercetin and kaempferol and we hypothesized that the blockage in the mutant occurred between either dihydroflavonols and leucoanthocyanidin or between leucoanthocyanidin and the end product anthocyanin. Acid hydrolysis and acid addition were used to quantify and qualify spectral characteristics of intermediates. Aqueous phase extracts of leaves demonstrated the end product was most likely an anthocyanin because of red color, absorption at 530 nm and miscibility with amyl alcohol. Mutants and wild-type plants in Sarracenia purpurea and S. rubra ssp. jonesii are capable of producing proanthocyanidins while only wild-type contain pseudobase. Sarracenia purpurea mutants had a significantly greater accumulation of proanthocyanidins than wild-type while there was no such significant difference in S. rubra ssp. jonesii. These results suggest that the mutant is blocked in a late stage of anthocyanin biosynthesis between leucocyanidin and pseudobase. While mutants may accumulate precursors, additional investigation is needed. Sarracenia may serve as useful plants for research since the highly modified carnivorous and absorptive leaves may be fed suspected intermediates in this last step of anthocyanin synthesis. (D 1998 Elsevier Science Ireland Ltd. All rights reserved.
1. Introduction
The biochemical pathways for anthocyanin biosynthesis have been the subject of intense scientific investigation and many of the intermediates and enzymes involved have been isolated and characterized [1]. The pathway appears to be conserved among angiosperms [2] with variation in the affinity of enzymes in various species for substrate [3,4].
Pseudobases are the first detectable intermediates in mutants blocked between leucoanthocyanidin (3,4-cis diols) and anthocyanin [5]. Anthocyanins are in equilibrium with colorless pseudobases and a decrease in pH results in the red-colored anthocyanin predominating with a concomitant increase in absorbance at 530 nm [6,7]. A test for the presence of pseudobase is the immersion of a leaf in concentrated acidic media and observing an immediate change to red color. A classic test for the presence of proanthocyanidins in plants is a color change of the solution to red when heated under strong acidic conditions [8,9].
Romeo et al. [10] and Jay and Lebreton [11] detected the flavonols quercetin and kaempferol in Sarracenia. The dihydro forms of both of these compounds are known precursors for the respective flavonols as well as anthocyanins [12]. Romeo et al. [10] also detected both quercetin and kaempferol in the mutant S. purpurea f.
heterophylla. Kristiansen [13] found that [14C] dihydroquercetin is converted into leucocyanidins and procyanidins in barley. This established the relationship of leucocyanidins as precursors to both cyanidin and procyanidin. Other workers have also established that procyanidins are synthesized through the flavonoid/anthocyanin pathway [14-16].
The currently accepted pathway of dihydroflavonol metabolism to leucoanthocyanidin (via dihydroflavonol reductase) followed by leucoanthocyanidin conversion to pseudobase and on to anthocyanidins is therefore presumed to occur in Sarracenia. Green mutants of the normally red-leaved Sarracenia purpurea or S. rubra ssp. jonesii have been located in several locations and occur with wild-type plants [17-23]. Preliminary extraction procedures demonstrated the presence of red pigments absorbing at 530 nm in wild-type of both species but not in the mutants. The objective of this investigation was to elucidate the probable anthocyanin biosynthetic pathway in Sarracenia and to determine where the blockage occurred in mutant greens.
2. Materials and methods
2. 1. Experiment one
Six leaves of S. purpurea (Cooks Bayou, FL) and seven leaves of S. purpurea f. heterophylla (seed raised plants from Peter Pauts Nurseries) were collected from a research bog at Meadowview Biological Research Station in Caroline County, Virginia on 25 September 1994. The leaves were kept on ice for transportation from the research facility to the biology department at Virginia Commonwealth University. The leaves were then cleaned of insect debris, washed first with tap water and then distilled water, drained and weighed (Sarracenia purpurea (Cooks Bayou, FL) 34.8 g, and S. purpurea f. heterophylla 44.9 g). One hundred fifty-five and 200 mll of methanol were added to the respective specimens with a half quantity of water and leaves homogenized in a blender to a slurry. Solutions were allowed to sit in stoppered Erlenmeyer flasks at room temperature for 1 day. The slurry was then filtered on a Buchner funnel and the filtrate was reduced in vacuo with mild heat (50-60oC) to a third the volume. This resulted in 50 ml of S. purpurea and 60 ml of S. purpurea f. heterophylla solution. Solutions were then extracted three times with chloroform and the aqueous layer retained and after three days at room temperature was stored under refrigeration at 4oC. No change in color of solution was observed during this period.
A similar protocol was used on 32.5 g of leaves of S. rubra ssp. jonesii (wild-type; Pickens Co., SC) and 43.4 g of S. rubra ssp. jonesii (mutant green; Etowah, Henderson Co., NC) in October 1994 without reduction under heat and vacuum.
On 3 and 20 October 1994, a Varian DMS 80 UV/Vis. spectrophotometer was used to scan the respective aqueous solutions from 200-800 nm. Scan speed was 100 nm/min and quartz cuvettes were used with a methanol/water blank.
On 26 February 1995, 50 ml of the S. purpurea f. heterophylla solution was removed from the refrigerator and 8 ml of 12 N HCI was added to make a 2 N solution. The solution was placed in a round bottomed flask and boileezers added. A reflux system was set-up on a hot plate and the solution was boiled at 84oC for 15 min. After the solution cooled, it was filtered through a Buchner funnel and the filtrate scanned on the spectrophotometer.
2.2. Experiment two
On 18 June 1996, six leaves from one clone each of S. purpurea f. heterophylia (seed raised plant from material from Peter Pauls Nursery, NY) and S. purpurea (Bethel, DE) were collected at Meadowview Biological Research Station and stored overnight wrapped in a moist paper towel in a plastic bag in the refrigerator. The following day necroted areas were cut out of the leaves, leaves rinsed, drained and weighed. The upper half of each leaf was then cut into strips and immersed in 10 ml of HPLC grade methanol in a test tube. Test tubes were sealed and placed in a refrigerator. On 20 June 1996, samples were centrifuged at 10,000 rpm for 10 min and the supernatant was retained. On 21 June 1996, absorbance at 530 nm was measured on the spectrophotometer. Four ml of 4 N HCL were added to each test tube resulting in a change in pH from 7 to 2. After addition of the acid, precipitation and cloudiness was noted in all test tubes and additional centrifugation for 10 min at 10000 rpm was done. The supernatant was retrieved and the pellet discarded. Absorbance at 530 nm was determined on the spectrophotometer and test tubes returned to the refrigerator. On 26 June 1996, the test tubes were heated in a water bath at 84oC for 1 h followed by centrifugation at 10,000 rpm for 10 min and refrigerated. On 27 June 1996, absorption at 530 nm was recorded on the spectrophotometer for all samples. A similar procedure was performed on both mutant and wild-type S. rubra ssp. jonesii from 1-3 July 1996. A pooled t-test for independent samples with equal variance was performed on the net change in absorbance at 530 nm after acid hydrolysis and on leaf weight.
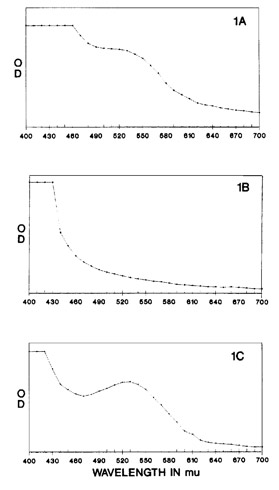
Fig. 1. Aqueous extracts of wild-type Sarracenia are characterized by red color and a peak at 530 nm (A) which is lacking in mutants (B). When the mutant aqueous extracts are boiled with acid colorless proanthocyanidins are
cleaved resulting in colored anthocyanidins with a peak at 530 nm (C).
3. Results
3.1. Experiment one
Aqueous extracts of S. purpurea from Cooks Bayou have a spectral peak at 530 nm (Fig. 1A) and solutions are reddish-purple colored while S. purpurea f. heterophylla (Peter Pauls) extracts lack such a peak and solutions are amber in color (Fig. 1B). Aqueous extracts of wild-type S. rubra ssp. jonesii were also red in color with a spectral peak at 530 nm while extracts of the green mutant were yellow-amber and lacked the peak at 530 nm. Addition of concentrated HCI to S. purpurea f. heterophylla creating a 2 N solution did not change the original amber color of this solution. Heating the acidified solution at 84oC resulted in a darkening of the solution within 5 min and a deep burgundy color within 15 min at which time boiling was stopped. The color change was also associated with a sharp increase in absorbance at 530 nm (Fig. 1C).
3.2. Experiment two
Methanolic extracts of mutant and wild-type S. purpurea and Sarracenia rubra ssp. jonesii were green in color. Mutant and wild-type did not have a peak of absorption at 530 nm due to the neutral pH of the methanolic solution.
The addition of 4 N HCI and adjustment of the solution to a pH of 2 resulted in a sharp increase in absorbance at 530 nm for wild-types in both species but not in mutants (Fig. 2). Color of wild-type extracts also changed from green to red as soon as acid was added while no color change was observed in mutants. Acidification of extracts resulted in denaturation and precipitation of chlorophyll which subsequently required centrifugation for spectrophotometric analysis.
Boiling with acid resulted in a change in the color of mutant solutions from green to orange or red and an increase in absorbance at 530 nm. Wild-type solutions intensified in red color with an increase in absorption at 530 nm as well (Fig. 2). The net change in absorption at 530 nm was significantly greater for the mutant in S. purpurea (mean = 1.14, S.E.M = 0.01, d.f. = 6) than wild- type (mean = 0.68, S.E.M = 0.02, d.f. = 6) while in S. rubra ssp. jonesii there was no significant difference between mutant (mean = 0.86, S.E.M = 0.01, d.f. = 5) and wild-type (mean = 1.20, S.E.M. = 0.01, d.f. = 5). There was no significant difference in mean weight of leaf samples between mutants and wild-types in either species (data not shown).
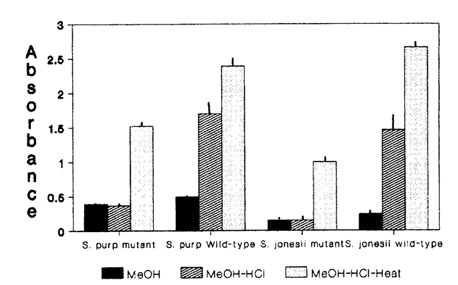
Fig. 2. Effect of methanol, acidification, and acid-boiling on absorbance at 530 nm in two mutant and wild-type Sarracenia species.
4. Discussion
Sarracenia is a genus of insectivorous pitcher plants restricted to wet, sunny, generally acid, nutrient poor habitats of the southeastern United States and Canada [241. They are herbaceous, rhizomatous plants that have leaves and stems modified into tubular or funnel shaped structures for catching and digesting insects. Brilliant coloration of leaves along with nectar and scent are thought to aid in trapping insects. [25]
Eaton [26] distinguished the species S. heterophylla from the red-pigmented S. purpurea because of its yellow corolla, overall yellow color and slender leaves. It was subsequently reduced to a form status by Fernald [27] and Wherry [28] considered it an anthocyanin-free mutation. This and similar mutants have now been found in five species at a variety of locations over the past 50 years [19,22] occurring singly or as a few individuals intermixed with normal wild-type plants [18,21,22]. The term 'green' was proposed [20,23] in place of Wherry's 'anthocyanin-free mutation' for brevity and to highlight the fact that no anthocyanin had yet been isolated from wild-type plants and was thus lacking in mutants. Greens are also recessive to the dominant wild-type red allele [20,23,29].
Proanthocyanidins (sic leucoanthocyanidins) and anthocyanins are water soluble compounds. The presence of anthocyanins is indicated in pitcher plant leaves because of the red color, absorption at 530 nm and aqueous nature of wild-type Sarracenia leaf extracts. The red color change and increase in absorbance at 530 nm in aqueous extracts of S. purpurea f. heterophylia after heating with acid are indicative of the presence of proanthocyanidins in this mutant. Methanolic extracts of S. purpurea and S. rubra ssp. jonesii mutant and wild-type also increase in absorption at 530 nm after heating with acid demonstrating the presence of proanthocyanidins in both species and phenotypes. Jay and Lebreton [11] report leucocyanidins (procyanidins) as well in several wild-type Sarracenia species. We have also found that after acid hydrolysis, methanolic extracts of Sarracenia produce a red precipitate insoluble in water but soluble in amyl alcohol. Solubility in amyl alcohol is a characteristic of anthocyanidins. Anthocyanidins are produced from the acid hydrolysis of procyanidins and this would tend to confirm that both mutants and wild-type are capable of producing proanthocyanidins.
The change in color from green to red observed in experiment two with wild-type S. purpurea and S. rubra ssp. jonesii as well as the large increase in absorbance at 530 nm suggests that pseudobases occur in wild-type plant extracts. Similarly the lack of such a change in mutant S. purpurea and S. rubra ssp. jonesii indicate the lack of pseudobase in these mutants.
Green mutant Sarracenia are not caused by a lesion in the dihydroflavonol reductase (DFR) gene because Murray and Hackett [4] demonstrated that Hedera plants lacking DFR activity do not produce procyanidins as detected by acid hydrolysis. Green mutant Sarracenia, however, are capable of producing procyanidins. Since proanthocyanidins are naturally produced by both wild-type and green mutant Sarracenia, the green mutation must be between the leucoanthocyanidin and anthocyanidin step in the flavonoid pathway (Fig. 3).
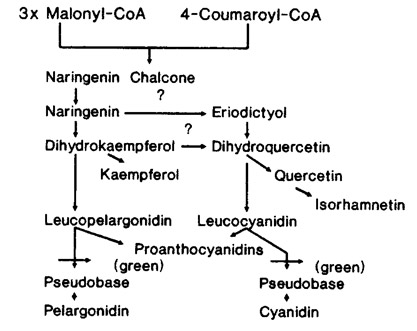
Fig. 3. A partial schematic of the flavonoid biosynthetic pathway in Sarracenia. The green mutant is blocked between leucoanthocyanidin and the end product anthocyanin.
References
[1] B. Jordan, The Molecular Biology of Flowering, CAB International, Wallingford, UK, 1993.
[2] F. Sparvoli, C. Martin, A. Scienza, G. Gavazzi, C. Tonelli, Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.), Plant Mol. Biol. 24 (1994) 743- 755.
[3] G. Forkmann, B. Ruhnau, Distinct substrate specificity of dihydroflavonol 4-reductase from flowers of Petunia hybrida Z, Naturforsch. 42 (1987) 1146-1148.
[4] J.R. Murray, W.P. Hackett, Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L, Plant Physiol. 97 (1991) 343-351.
[5] W. Heller, G. Forkmann, Biosynthesis, in: J.B. Harborne (Ed.), The Flavonoids, Advances in Research Since 1980, Chapman&Hall, New York, 1988, pp. 399-425.
[6] R. Brouillard, The in vivo expression of anthocyanin colour in plants, Phytochemistry 22 (1983) 1311-1323.
[7] R.D. Curtright, J.A. Rynearson, J. Markwell, Fruit anthocyanins, colorful sensors of molecular milieu, J. Chem. Educ. 71 (1994) 682-684.
[8] J.B. Harbome, T.J. Mabry, H. Mabry, The Flavonoids, Chapman & Hall, London, 1975.
[9] T. Robinson, The Organic Constituents of Higher Plants, Their Chemistry and Interrelationships, Cordus Press, MA, 1980.
[10] J.T. Romeo, J.D. Bacon, T.J. Mabry, Ecological considerations of amino acids and flavonoids in Sarracenia species, Biochem. Syst. Ecol. 5 (1977) 117-120.
[11] M. Jay, P. Lebreton, Chemotaxonomic research on vascular plants 26. The flavonoids of the Sarraceniaceae, Nepenthaceae, Droseraceae and Cephalotaceae; a critical study of the order Sarraceniales, Naturaliste Canadienne 99 (1972) 607-613.
[12] K.E. Schwinn, K.E. Markham, N.K. Given, Floral flavonoids and the potential for pelargonidin biosynthesis in commercial chrysanthemum cultivars, Phytochemistry 35 (1994) 145-150.
[13] K.N. Kristiansen, Biosynthesis of proanthocyanidins in barley: genetic control of the conversion of dihydroqueretin to catechin and procyanidins, Carslberg Res. Commun. 49 (1984) 503-524.
[14] D. Jacques, C.T. Opie, L.J. Porter, E. Haslam, Plant proanthocyanidins. Part 4. Biosynthesis of procyanidins and observations on the metabolism of cyanidin in plants, J. Chem. Soc. Perkin Trans. 1 (1977) 1637-1643.
[15] H.A. Stafford, M. Shimarnoto, H.H. Lester, Incorporation of [14 C] phenylalanine into flavan-3-ols and pro-
cyaniding in cell suspension cultures of Douglas fir, Plant Physiol. 69 (1982) 1055-1059.
[16] M. Zaprometov, H. Grisebach, Dihydroflavonols as precursors for catechins in the tea plant, Z. Naturforsch. 28 (1973) 113-115.
[17] C.R. Bell, A cytotaxonomic study of the Sarraceniaceae of North America, J. Elisha Mitchell Sci. Soc. 65 (1949) 137-166.
[18] F. Case, Some Michigan records for Sarracenia purpurea forma beterophylla, Rhodora 58 (1956) 203-207.
[19] J.T. Robinson, Sarracenia purpurea L. forma heterophylia (Eaton) Fernald: new to Connecticut, Rhodora 83 (1981) 156-157.
[20] P. Sheridan, Genetics of Aberrant Sarracenia Leaf and Flower Color, Virg. J. Sci. 45 (1994) 71.
[21] P. Sheridan, B. Scholl, Sarracenia purpurea ssp. purpurea f. heterophylla (Eaton) Fernald in Nova Scotia, Camiv. Plant Newslett. 22 (1993) 106-107.
[22] P. Sheridan, B. Scholl, Noteworthy Sarracenia collections, Carniv. Plant Newslett. 22 (1993) 58-61.
[23] P. Sheridan, B. Scholl, Noteworthy Sarracenia collections 11, Carniv. Plant Newslett. 25 (1996) 19-23.
[24] F.E. Lloyd, The Carnivorous Plants, Chronica Botanica, Waltham, MA, 1942, pp. 1-352.
[25] B.E. Juniper, R.J. Robins, D.M. Joel, The Carnivorous Plants, Academic Press, New York, 1989, pp. 1-353.
[26] A. Eaton, Manual of Botany for the Northern and Middle States of America, Webster and Skinners, Albany, 1822, pp. 447-448.
[27] M.L. Fernald, Notes on the flora of western Nova Scotia, Rhodora 24 (1922) 165-183.
[28] E. Wherry, The geographic relations of Sarracenia purpuree, Bartonia 15 (1933) 1-8.
[29] P. Sheridan, Genetics of Sarracenia leaf and flower color. Part 1, Camiv. Plant Newslett. 26 (1997) 51-64.